Batteries – everything you need to know

In our rapidly evolving world, batteries have become an integral part of our daily lives, revolutionizing the way we use and store energy. From powering our smartphones to driving electric vehicles and even storing renewable energy, batteries have come a long way since their invention. In this blog post, we will delve into the extensive world of batteries, exploring their history, their basic functioning, and their differences.
When were batteries invented? – A historical review
The history of batteries can be traced back to the late 18th century when Italian physicist Alessandro Volta invented the “Voltaic Pile,” the first electrochemical battery. He used copper and zinc plates separated by brine-soaked paper disks to generate a steady current. However, Volta misunderstood the source of voltage, and it was not until Michael Faraday’s work in 1834 that the true nature of the cells was understood.
Over the years, battery technology has evolved significantly, with scientists and engineers constantly pushing the boundaries to improve efficiency and performance. For example, the early batteries, while useful for experimentation, did not have stable voltages and sustained power output. The first battery was not used industrially until the Daniell cell (invented in 1836) – mainly to supply telegraph networks. However, Daniell cells were prone to leakage and breakage due to their liquid electrolytes and glass vessels, making them unsuitable for portable applications. As a solution, the dry battery was introduced at the end of the 19th century, viable for portable electrical devices.
Both wet and dry batteries are still used today, albeit usually in modified form. Common examples for dry cell batteries are Alkaline or Lithium-ion batteries typically used in handheld and portable devices. An adopted example of a wet cell battery is the lead-acid gel battery, which is still frequently used as a car battery or for the emergency power supply of buildings.
How do batteries work? – The operating principle
Put simply, batteries convert chemical energy into electrical energy due to an electrochemical reaction of metal, oxides, or molecules. We will explain this reaction based on the operating principle of a voltaic (or galvanic) cell as a classical battery consists of multiple voltaic cells.
Note: Voltaic cells are also referred to as galvanic cells and are based on the same functional principle. Both terms are in fact used since Luigi Galvani and Alessandro Volta both played an essential role in the invention of the first battery.
In its simplest form, a single voltaic cell is composed of two half-cells connected by a semi-permeable membrane or a salt bridge. Each half-cell consists of a metal (electrode) immersed in a solution containing the respective metal ions (electrolytes). One half cell contains a negative electrode (anode) which attracts anions (negatively charged ions), whereas the other half cell contains a positive electrode (cathode) which attracts cations (positively charged ions).
Example based on a Zn-Cu voltaic cell:
Figure 1 displays a schematic representation based on a zinc-copper (Zn-Cu) voltaic cell. The blue half cell shows the Zn electrode (anode) placed in a solution of ZnSO4 (zinc sulfate). The green half cell contains the Cu electrode (cathode) in a solution of CuSO4 (copper sulfate). A salt bridge connects the sulfates. If we now complete the electric circuit via an external electric conductor, Zn2+ ions dissolve from the Zn electrode into the solution (oxidation) and electrons enter the external conductor. In order to compensate for the increased zinc ion concentration, zinc ions leave and anions enter the zinc half cell via the salt bridge. In the copper half cell, the Cu2+ ions (cations) attach themselves to the Cu electrode (reduction) and take up electrons from the outer conductor.
This chemical reaction can be described by means of the following reduction-oxidation (redox) equation:
Zn(s) + Cu2+(aq) –> Zn2+(aq) + Cu(s)
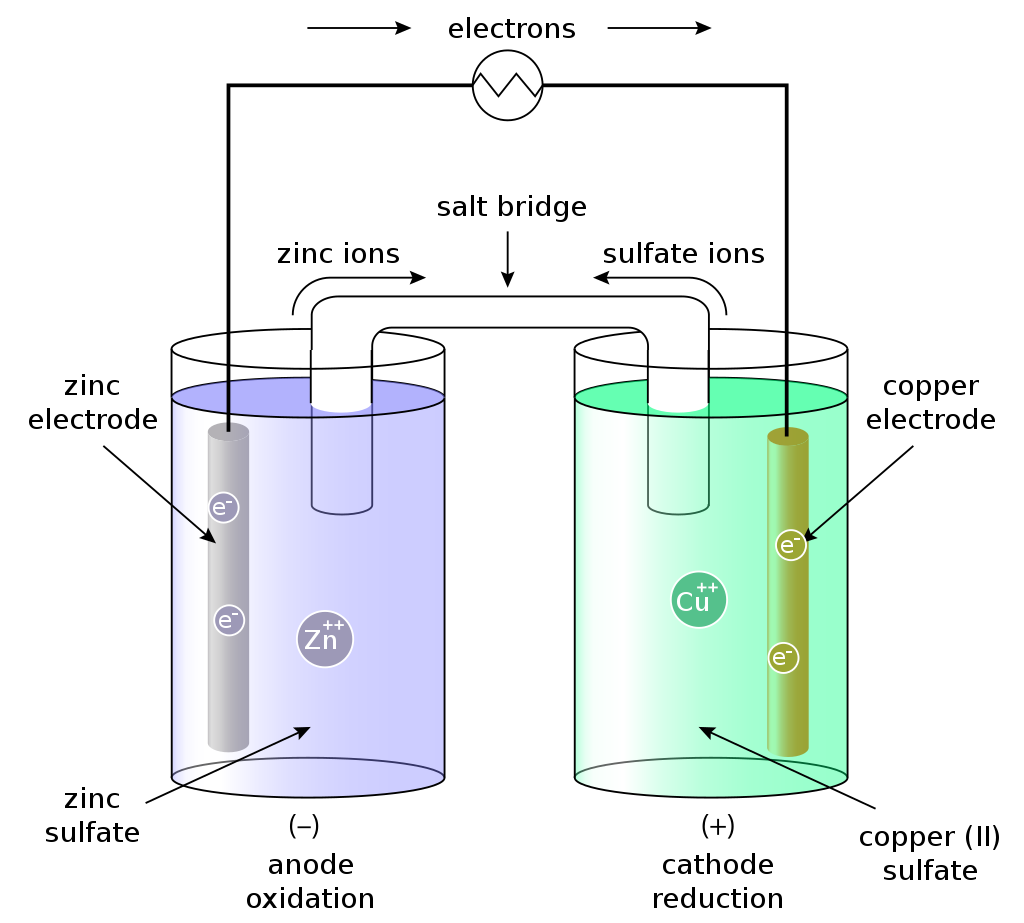
Figure 1: Schematic of a zinc-copper (Zn-Cu) voltaic cell. (©Rehua)
What battery types are there? – Primary vs. secondary
Earlier, we distinguished batteries based on the state of matter of the electrolyte (wet cells, gel cells, dry cells). However, batteries are also classified depending on their rechargeability. In general, we distinguish between primary and secondary batteries.
Primary batteries
Primary batteries are designed to be used until energy is exhausted. Their chemical reactions are usually not reversible, therefore they cannot be recharged. As soon as the supply of reactants in the battery is exhausted, the battery no longer produces electricity and is unusable.
Secondary batteries
Secondary batteries are rechargeable, i.e. their chemical reactions can be reversed by applying an electric current to the cell. This regenerates the original chemical reactants so that they can be used again multiple times.
The following table summarizes and compares some basic information regarding primary and secondary batteries:
| Primary batteries | Secondary batteries | |||
| Rechargeability | Non-rechargeable, designed for single-use | Rechargeable, can be used multiple times | ||
| Energy density | Generally have a higher initial energy density, which means they can store more energy in a given volume. | Slightly lower initial energy density | ||
| Lifespan | Often have a longer shelf life than secondary batteries, but due to non-rechargeability service lifespan is way shorter. | Number of charge-discharge cycles depends on chemical composition and application, however longer service lifespan than primary batteries | ||
| Application | Commonly used in devices where consistent power is needed for a limited time and where recharging is not feasible or practical: smoke detector, remote-control, … | Preferred for devices that require frequent use and where recharging is convenient: smartphones, laptops, cars, … | ||
| Examples | Zink-carbon, alkaline, lithium-silicon, … | Lead-acid, lithium-ion, nickel-cadmium, … |
What are the key characteristics of a battery? – Properties and more
In general, a battery’s key characteristics include the battery capacity, its discharge rate, and its overall performance. They may vary due to many factors regarding load cycle, charge cycle, and lifetime. The most important ones are internal chemistry, current drain, and temperature. Depending on the application of your batteries, it is essential to monitor or regularly check their properties – whether for electric vehicles in the automotive industry, for battery storage power plants in the energy industry, or for other applications.
Therefore, we at DEWETRON provide you with several solutions to measure and analyze the characteristics of your battery, such as:
- cell characterization at different load profiles and different temperatures,
- long-term performance and aging tests,
- module and system level testing regarding the interaction of the whole battery system and battery management,
- checks such as overcharging, short-circuit, mechanical damage, and overheating.
Explore our product range, choose your ideal measurement tools and start your analysis with our intuitive measurement software OXYGEN. If you are interested or simply have some unanswered questions, contact us directly.